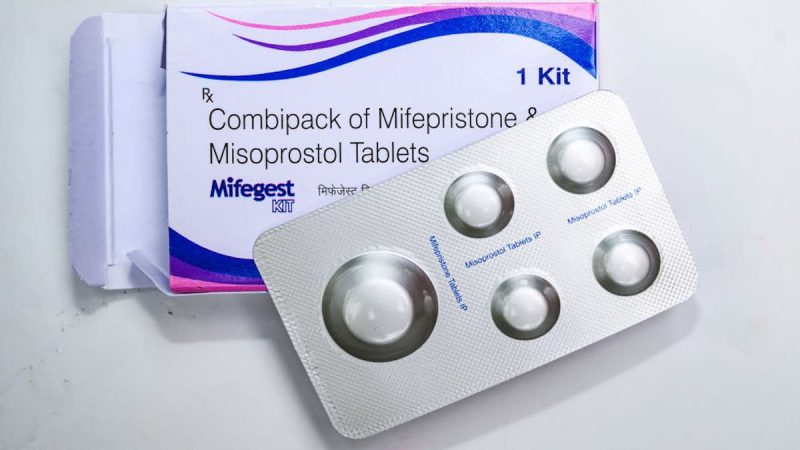
The Supreme Court will hear arguments in a case challenging access to the abortion pill and its regulatory approval process on March 26, the court announced Monday.
In December, the nation’s highest court agreed to consider appeals from the Biden administration and drug manufacturer Danco defending several moves by the U.S. Food and Drug Administration (FDA) intended to make it easier to access and use the mifepristone pill in the wake of the overturning of Roe v. Wade last year.
In overturning Roe v. Wade in June 2022, the Supreme Court ruled in Dobbs v. Jackson Women’s Health Organization that the U.S. Constitution does not guarantee the right to an abortion and that the matter may be decided by the states.
In the aftermath, 14 states have banned abortion at all stages of pregnancy, with some exceptions, and two others have banned abortion once a fetal heartbeat is detected, which is around six weeks of gestation.
The Biden administration and the maker of the drug mifepristone are asking the high court to reverse an appellate ruling that would cut off access to the drug through the mail and impose other restrictions, even in states where abortion remains legal.
The restrictions include shortening from the current 10 weeks to seven weeks, the time during which mifepristone can be used in pregnancy. The nine justices rejected a separate appeal from abortion opponents who challenged the FDA’s initial approval of mifepristone as safe and effective in 2000.
Erin Hawley, counsel for the civil rights firm Alliance Defending Freedom challenging the Biden administration, accused the Biden administration of ‘defending the FDA’s reckless removal of the safety standards it originally deemed necessary for women who use abortion drugs.’
‘The FDA’s own label for these drugs says that roughly one in 25 women who take them will end up in the emergency room. The agency’s removal of in-person doctor visits and consistent, ongoing care has subjected more women to suffering severe, even life-threatening, medical conditions,’ Hawley said in a statement.
‘Regardless of Americans’ beliefs about abortion, no one should be okay with the FDA leaving girls to take these high-risk drugs all alone,’ she added.
The Supreme Court will hear arguments in the case called FDA v. Alliance for Hippocratic Medicine on Tuesday, March 26 at 10:00 a.m.